COVID-19 IgM/IgG Rapid Testing Antibody Kit
COVID-19/ SARS-CoV-2 (Corona Virus Disease) is an infectious disease caused by the most recently discovered coronavirus. COVID-19/ SARS-CoV-2IgG/IgM Rapid Test (Serum/Plasma/Whole Blood) is a rapid chromatographic immunoassay for the qualitative of IgG and IgM antibodies to COVID-19 in human serum, plasma or whole blood. Advantages of the product 1) Early discovery: positive IgM indicate early phase of infection 2) Whole spectrum: cover early, mid and late phase of disease, avoid missing 3) Efficiency: one test contain specific IgM and IgG antibodies, don’t need any other equipment, and the result can be read in ten mins. 4)Safety: Individual test avoid cross-infection.
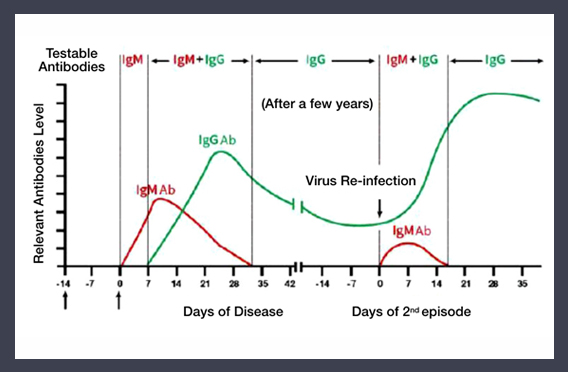
IgM antibody: Appear on day 1-7 of an acute infection. Detection of IgM in blood indicates a recent infection. It can be used as early screening for SRAS- Cov-2 suspected population.
IgG antibody: In the mid to late stage of infection, B lymphocytes enter lymph node and transform into plasma cells and produce large amount of IgG. Detection of IgG indicate on-going or past infection, useful in the monitoring of progress of infection.
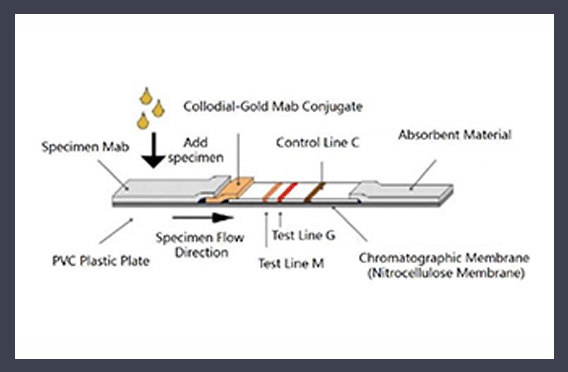
The outbreak of the novel coronavirus (SARS-CoV-2) disease is now a pandemic and a serious public health crisis worldwide. There is an urgent need for the introduction of an accurate, easy, and rapid alternative test to identify potential infected individuals. The Diagnostic Kit for Antibody IgM/IgG of Novel Coronavirus COVID-19 was prepared by colloidal gold immunochromatography using anti-human IgM and anti-human IgG antibodies coated on nitrocellulose membranes and colloidal gold labeled SARS-CoV-2 S/N antigens and other reagents.
When tested, the gold-labeled antigen will form a complex with the specific antibody in the sample. If there is a IgM or IgG antibody specific to the SARS-CoV-2 antigen in the sample, the antibody forms a complex with the gold-labeled antigen and moves forward along the test strip due to chromatography, and the solid-phase anti-human IgM or anti-human antibody in the detected line is captured to form a sandwich complex to condense the color.
If it is a negative specimen, even if it can form a sandwich complex, it can not be captured as a specific antigen component in the detection area, so it does not develop color. Regardless of whether there is SARS- CoV-2 antibody in the sample, a colored strip will appear in the quality control area, as the internal control standard of whether the chromatography process is normal or not, and whether the reagent is invalid.
Storage Conditions
- The kit should be stored at 2℃~30℃, sealed,avoid direct sunlight and the validity period is 18 months.
- The test card should be used within 1 hour after taking out from the foil pouch.
- Specimen diluent should be used within 14 days after opened and stored at 2℃~30℃.

Sensitivity: A/ ( A+C ) ×100%; 170/ (170+18) ×100%=90.43
Specificity: D/ ( B+D ) ×100%; 182/ ( 182+0)×100%=100
Total coincidence rate: = ( A+D) / ( A+B+C+D ) ×100%
= ( 170+182 )/( 170+182+18+0 )=95.14
Important Note
Negative results do not preclude acute SARS-CoV-2 infection. If acute infection is suspected, direct testing for SARS-CoV-2 is necessary.
Results from antibody testing should not be used to diagnose or exclude acute SARS-CoV-2 infection.
Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
This test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 (COVID-19) infection or to inform infection status.